|
Size: 1600
Comment:
|
← Revision 4 as of 2013-07-18 20:33:25 ⇥
Size: 3950
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 1: | Line 1: |
| ## page was renamed from [BimetalStripStand | |
| Line 2: | Line 3: |
| ||<:30%>[[PiraScheme#Thermodynamics| Table of Thermodynamics Demonstration]]||<:30%>[[TDEquipmentList| Thermodynamics Equipment List]]||<:30%>[[Demonstrations|Lecture Demonstrations]]|| | ||<30% style="text-align:center">[[PiraScheme#Thermodynamics|Table of Thermodynamics Demonstration]] ||<30% style="text-align:center">[[TDEquipmentList|Thermodynamics Equipment List]] ||<30% style="text-align:center">[[Demonstrations|Lecture Demonstrations]] || |
| Line 5: | Line 7: |
| '''Topic and Concept:''' | |
| Line 6: | Line 9: |
| '''Topic and Concept:''' Thermal Properties of Matter, [[ThermalProperties#SolidExpansion| 4A10. Solid Expansion]] |
Thermal Properties of Matter, [[ThermalProperties#SolidExpansion|4A30. Solid Expansion]] |
| Line 10: | Line 12: |
| Line 11: | Line 14: |
| * '''Bay:''' [[ThermoCabinetBayA1|(A1)]] * '''Shelf:''' #1,2,3.. |
* '''Bay:''' [[ThermoCabinetBayA1|(A1)]]? * '''Shelf:''' #1,2,3..? |
| Line 14: | Line 17: |
| attachment: mainPhoto | {{attachment:BimetalStripStand01-400.jpg}} |
| Line 18: | Line 21: |
| Insert succinct description of demonstration. | A mounted bimetallic strip is heated or cooled and compared to its room temperature state. |
| Line 20: | Line 23: |
| ||<:style="width: 60%" :40%>'''Equipment'''||<:30%>'''Location'''||<:25%>'''ID Number'''|| | ||<40% style="text-align:center">'''Equipment''' ||<30% style="text-align:center">'''Location''' ||<25% style="text-align:center">'''ID Number''' || |
| Line 22: | Line 25: |
| ||apparatus||[[ThermoCabinetBayB1| TD, Bay B1, Shelf #2]]|| || ||all other parts||[[ThermoCabinetBayB1| TD, Bay B1, Shelf #2]]|| || ||...||[[ThermoCabinetBayA5| TD, Bay A5, Shelf #2]]|| || |
||Mounted bimetallic strip||[[ThermoCabinetBayB1|TD, Bay B1, Shelf #2]] ??|| || ||Burner with attached air hose ||[[ThermoCabinetBayB1|TD, Bay B1, Shelf #2]] ??|| || ||[[RedWhiteGasCart|Red and white gas carts]]||Rooms 2103, 2241, (and 2223 upon request)|| || ||Liquid nitrogen|| || || || Safety glasses and gloves|| || || |
| Line 27: | Line 32: |
| '''''Important Setup Notes:''''' * ''''' ''''' |
'''''Important Setup Notes:''''' * This demonstration requires a supply of methane gas usually provided by the [[RedWhiteGasCart|red and white gas carts]] found in rooms 2103, 2241, (and 2223 upon request). * This demonstration requires a supply of liquid nitrogen. The main supply is located at the loading dock. If 12 hours notice given to lecture demo, supply will be provided. |
| Line 31: | Line 38: |
| '''Setup and Procedure:''' | '''Setup and Procedure:''' |
| Line 33: | Line 40: |
| 1. List steps for setup then procedure. 1. ... |
1. Set the red needled so that it marks the position of the end of the bimetallic strip. 1. To light the burner, connect the attached gas hose to the gas out (red panel) on the [[RedWhiteGasCart|red and white gas cart]]. 1. Open the gas valve. 1. Light a match and bring it near the top of the burner. 1. The flame will ignite the gas. Adjust the flame height accordingly by adjusting the valve. 1. Use the lit flame to heat the bimetallic strip by hand and notice which way the strip curls. 1. Extinguish the flame by closing the gas valve. 1. Pour liquid nitrogen on the strip to cool it and notice that the strip curls in the opposite direction. |
| Line 37: | Line 50: |
| * | * Always use the gloves and safety glasses throughout this demonstration. * Beware of the heated plate - contact with skin could cause severe burns! * Use care when working with the liquid nitrogen - prolonged contact with skin causes severe frostbite! '''Discussion:''' The bimetallic strip consists of two different metals. Since one metal has a higher coefficient of thermal expansion than the other, it expands more for a given change in temperature than does the other. Thus, when heated, the strip bends toward the side with the lower coefficient of thermal expansion. On the other hand, when the strip is placed in liquid nitrogen (77K), the same side with a higher coefficient contracts more for a given change in temperature than does the other metal causing the strip to bend in the opposite direction. ||{{attachment:BimetalStripStand01-250.jpg}}||{{attachment:BimetalStripStand02-250.jpg}}||{{attachment:BimetalStripStand03-250.jpg}}|| |
| Line 40: | Line 61: |
| '''Discussion:''' | |
| Line 42: | Line 62: |
| Discuss the physics behind the demonstration, explaining some of the various steps of the demonstration when appropriate. ||attachment: photo||attachment: photo||attachment: photo||attachment: photo|| |
|
| Line 47: | Line 64: |
| Line 50: | Line 68: |
* List any references |
* [[http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/bimetal.html|Hyperphysics - Bimetalic Strip]] * [[https://en.wikipedia.org/wiki/Thermal_expansion|Wikipedia - Thermal Expansion]] * [[http://en.wikipedia.org/wiki/Coefficient_of_thermal_expansion|Wikipedia - Coefficient of Thermal Expansion]] |
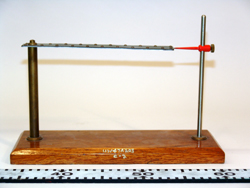
Bimetallic Strip with Stand, 4A30.10b
Topic and Concept:
Thermal Properties of Matter, 4A30. Solid Expansion
Location:
Cabinet: Thermodynamics (TD)
Bay: (A1)?
Shelf: #1,2,3..?

Abstract:
A mounted bimetallic strip is heated or cooled and compared to its room temperature state.
Equipment |
Location |
ID Number |
|
|
|
Mounted bimetallic strip |
|
|
Burner with attached air hose |
|
|
Rooms 2103, 2241, (and 2223 upon request) |
|
|
Liquid nitrogen |
|
|
Safety glasses and gloves |
|
|
Important Setup Notes:
This demonstration requires a supply of methane gas usually provided by the red and white gas carts found in rooms 2103, 2241, (and 2223 upon request).
- This demonstration requires a supply of liquid nitrogen. The main supply is located at the loading dock. If 12 hours notice given to lecture demo, supply will be provided.
Setup and Procedure:
- Set the red needled so that it marks the position of the end of the bimetallic strip.
To light the burner, connect the attached gas hose to the gas out (red panel) on the red and white gas cart.
- Open the gas valve.
- Light a match and bring it near the top of the burner.
- The flame will ignite the gas. Adjust the flame height accordingly by adjusting the valve.
- Use the lit flame to heat the bimetallic strip by hand and notice which way the strip curls.
- Extinguish the flame by closing the gas valve.
- Pour liquid nitrogen on the strip to cool it and notice that the strip curls in the opposite direction.
Cautions, Warnings, or Safety Concerns:
- Always use the gloves and safety glasses throughout this demonstration.
- Beware of the heated plate - contact with skin could cause severe burns!
- Use care when working with the liquid nitrogen - prolonged contact with skin causes severe frostbite!
Discussion:
The bimetallic strip consists of two different metals. Since one metal has a higher coefficient of thermal expansion than the other, it expands more for a given change in temperature than does the other. Thus, when heated, the strip bends toward the side with the lower coefficient of thermal expansion. On the other hand, when the strip is placed in liquid nitrogen (77K), the same side with a higher coefficient contracts more for a given change in temperature than does the other metal causing the strip to bend in the opposite direction.
|
|
|
Videos:
References: