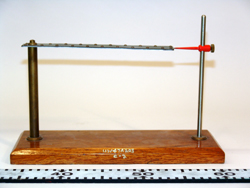
Bimetallic Strip with Stand, 4A30.10b
Topic and Concept:
Thermal Properties of Matter, 4A30. Solid Expansion
Location:
Cabinet: Thermodynamics (TD)
Bay: (A1)?
Shelf: #1,2,3..?

Abstract:
A mounted bimetallic strip is heated or cooled and compared to its room temperature state.
Equipment |
Location |
ID Number |
|
|
|
Mounted bimetallic strip |
|
|
Burner with attached air hose |
|
|
Rooms 2103, 2241, (and 2223 upon request) |
|
|
Liquid nitrogen |
|
|
Safety glasses and gloves |
|
|
Important Setup Notes:
This demonstration requires a supply of methane gas usually provided by the red and white gas carts found in rooms 2103, 2241, (and 2223 upon request).
- This demonstration requires a supply of liquid nitrogen. The main supply is located at the loading dock. If 12 hours notice given to lecture demo, supply will be provided.
Setup and Procedure:
- Set the red needled so that it marks the position of the end of the bimetallic strip.
To light the burner, connect the attached gas hose to the gas out (red panel) on the red and white gas cart.
- Open the gas valve.
- Light a match and bring it near the top of the burner.
- The flame will ignite the gas. Adjust the flame height accordingly by adjusting the valve.
- Use the lit flame to heat the bimetallic strip by hand and notice which way the strip curls.
- Extinguish the flame by closing the gas valve.
- Pour liquid nitrogen on the strip to cool it and notice that the strip curls in the opposite direction.
Cautions, Warnings, or Safety Concerns:
- Always use the gloves and safety glasses throughout this demonstration.
- Beware of the heated plate - contact with skin could cause severe burns!
- Use care when working with the liquid nitrogen - prolonged contact with skin causes severe frostbite!
Discussion:
The bimetallic strip consists of two different metals. Since one metal has a higher coefficient of thermal expansion than the other, it expands more for a given change in temperature than does the other. Thus, when heated, the strip bends toward the side with the lower coefficient of thermal expansion. On the other hand, when the strip is placed in liquid nitrogen (77K), the same side with a higher coefficient contracts more for a given change in temperature than does the other metal causing the strip to bend in the opposite direction.
|
|
|
Videos:
References: