Freezing by Evaporation, 4C31.32
Topic and Concept:
Change of State, 4C31. Cooling by Evaporation
Location:
Cabinet: Thermodynamics (TD)
Bay: (A1)
Shelf: #1,2,3..

Abstract:
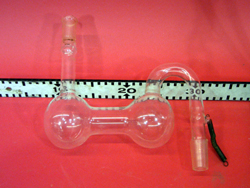
Freeze water in a flask by pumping through a sulfuric acid trap. Supercooling up to 10 C is possible
Equipment |
Location |
ID Number |
|
|
|
Vacuum pump |
Floor item: ME south wall |
|
Water |
|
|
Concentrated sulfuric acid (H2SO4) |
|
|
Glass apparatus |
|
|
Stand w/ clamp |
|
|
Important Setup Notes:
- This demonstration requires sulfuric acid.
Setup and Procedure:
- Fill the bottommost glass flask with about xxxx of water.
- Fill the higher two bulb flask with sulfuric acid.
- Mount the glass apparatus on the stand using the clamp.
- Connect the vacuum hose to the uppermost opening of the flask.
- Turn on the vacuum pump by flipping the on/off switch to the on position.
- Close the green valve to vacuum out the air in the tube and begin the demonstration of evaporative cooling.
Cautions, Warnings, or Safety Concerns:
- Sulfuric acid can cause severe chemical burns! Exercise care when handling, and wash any exposed skin immediately.
Discussion:
When we begin removing air from inside the glass vessel, we reduce the internal pressure. Quickly, the pressure drops low enough that both the acid and water exist as a gas. This is what causes the boiling. The now forming vapor carries away energy from the liquid causing the total internal energy of the liquid to decrease. Correspondingly, the temperature of the liquid water decreases until the triple point is reached. Continued boiling causes the temperature to drop further until the water is completely solid.
|
|
|
|
|
|
|
|
|
|
|
|
|
Videos:
References: